The plasma above is greenish. There are only a few causes of this–one is from iron metabolism. An increase in iron in the serum comes from somewhere–perhaps the stores of hemoglobin. Is this a clue? It is possible that viral proteins may attack the B1 subunit of hgb (1). This may effectively deactivate this subunit. If the virus does this to 100% of your hgb, this would leave you with an spO2 of 75%. It probably does not affect 100% and the sat we see is probably a combination of this and the shunting in the lungs, but this helps explain laughing on the phone at 75%.
With only three cooperative sites we are not taking advantage of the highest affinity binding site. This leaves us on the steep portion of the curve without our usual reserve and shifts the whole curve to the left as below. It also changes the shape of the sigmoid, less cooperation and steeper.
Whether the COVID sludge (frothy pulmonary edema) results from more of an ARDS or HAPE type pathphysiology is now an ongoing discussion and regardless will result in significant shunting. This shunting further lowers the PaO2 and diffusion impairment further decreases the PaO2 and increases the A-a gradient.
Increasing FiO2 will increase the pAO2 back to normal and help correct hypoxemia. But remember, even if we are doing this to reduce or eliminate shunt, the hgb is bound by the virus and 75% could be as good as it gets.
If you get a sat higher than 75% this implies not all hgb is affected. We don’t know what proportion may be. 50%, 100%, 15%? All would create different phenotypes and result in different dissociation curves as below. You can not use FiO2 to increase beyond YOUR hgb’s capacity but it also increases the paO2—the partial pressure of the O2 dissolved in the blood.
We know that higher hgb levels is associated with worse outcomes and men have higher hgb levels (women also have higher levels of possibly protective 2,3dpg).So the next series of questions are should we exchange transfuse. I believe the answer is no. If you transfuse more hgb (more m/m) and the viral protein binds it—it does not help and might hurt.
It might hurt because the viral—porphyrin moietey seems to allow the virus to more easily invade the fatty membranes of the epithelium anywhere, especially the alveoli. This potentially contributes to the prothrombotic effects which generally starts with endothelial injury.
If the cause of death is frequently complications from thrombosis then transfusion might make them more sick and promote thrombosis.
Perhaps leeches or bloodletting? Is anemia protective? HBO?
Does Thalassemia explain Italy? Glycosylated hgb appears to be more susceptible making it worse for diabetics and the elderly and also may cause labile glucose levels.
And does this make anticoagulation key?
The concern for hemoglobinopathy becomes clear when you look at allosteric enzyme binding. This is a graph of cooperative binding showing the same sigmoid curve as the hemoglobin dissociation curve.
Curve A shows no cooperativity—where four sites just bind equally without increasing affinity as you increase the number of sites bound. This curve is a hyperbole. Curve C has a much more gradual slope and is a sigmoid curve. This represents high cooperativity like hemoglobin.A pure hyperbole has a hill coefficient which represents cooperativity of zero. Hemoglobin has a hill coefficient of 2.7. Most of that number comes from the highest affinity finding which is the fourth oxygen. When this is blocked, the hemoglobin only has three cooperative sites
This lowers the hill coefficient. This leaves the graph of a three binding site hemoglobin closer to curve B above. Or if 50% of the hgb is poisoned, approximately a B+ curve between B and C. Regardless, this has a much steeper slope. This means that a patient can rapidly unbind all of its oxygen very rapidly. It represents poor physiologic reserve.
When we think of a shift left/right of the hgb dissociation curve this assumes the number of binding sites remains constant. With only 3, the curve migrates to the left. This is not the same as shifting. From this new baseline you can shift left or right based on local factors
Remember that the X axis is PaO2.
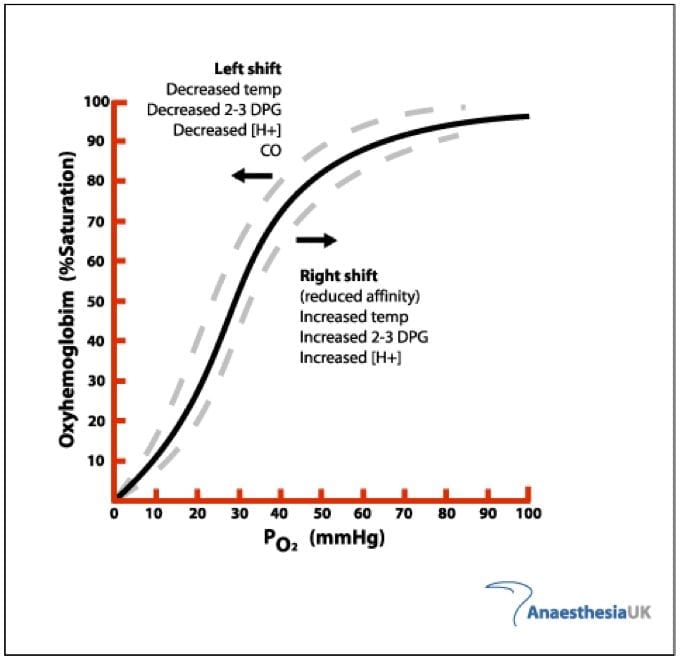
If you are breathing 21% oxygen the partial pressure of oxygen in the alveoli of healthy long is 100 mmHg. If we give someone 100% oxygen we can increase this five fold. This is the partial pressure that determines the PaO2 which should also be 100 mmHg.
As you can see from the first graph, you will remain 100% saturated until a very low PaO2. However, this new graph is designed for the three binding site hemoglobin and when it’s 100% saturated it’s really 75% on the pulse ox.
Of course a 75% on the pulse ox assumes the 100% of your hemoglobin has been attacked and complexed by the virus.
It’s probably less than 100% but we don’t know much about the time course at all.
The pulse ox that we observe, is certainly not entirely explained by this alone. There is shunting going on early in the course and as this worsens the pulse ox will also worsen. The scary readings we are seeing are a combination of these 2 effects. Hemoglobinopathy and shunting.
To keep patients from falling precipitously off the cliff, you need to maximize their PaO2, keeping him on the flat part of the curve. It’s hard to say for sure what fall off the cliff means, but it might mean tissue hypoxia, end organ dysfunction and arrhythmia.
We know we are seeing sudden death from thromboembolism, possibly also promoted by the heme complex, but it may not explain all of these events— this might explain a portion as well.
So proning, high FiO2, and other appropriate vent strategies are important to optimizing FiO2. Hyperbarics works well for this and it’s being discussed. Unfortunately we have far fewer hyperbaric chambers then we do ventilators. It’s possible The chamber could prevent intubation.
Anticogulation may be key to prevent thromboembolic events–probably the prime cause of mortality. So far TEG studies do not offer much optimism as COVID may be resistant to even full therapeutic dose heparin.
What about iron toxicity? I think thats exactly what we are seeing. Abdominal pain is not an uncommon presentation of COVID, including nausea, vomiting and diarrhea. Is this direct viral invasion? Few patients in the small data sets so far have had fecal viral shedding, although it has occurred. I think these are from iron toxicity. An adult make has about 16 grams of hgb/dl. This is 800 grams for 5L of blood. If half our hgb is involved, thats 1388 then divided by four as only 1/4 of the iron, that from the B1 is involved. Thus this is mild and would not be expected to cause fulminant failure or significant transaminitis. There is some cell-free hemoglobin but it’s contribution would be small.
Until the picture becomes crystal clear, these clinical hypotheses are important for generating targeted research to collect data to help pinpoint the true narrative of this disease.
We are collecting as we speak.

This recent scientific manuscript supports this hypothesis…
“COVID-19: Attacks the 1-Beta Chain of Hemoglobin and Captures the Porphyrin to Inhibit Human Heme Metabolism”
https://chemrxiv.org/articles/COVID-19_Disease_ORF8_and_Surface_Glycoprotein_Inhibit_Heme_Metabolism_by_Binding_to_Porphyrin/11938173/5
LikeLike
Furthermore, there’s a really interesting manuscript posted a few weeks back in which researchers performed modeling in an attempt to re-purpose existing drugs. This work identified a number of drugs that target various viral proteins…
https://www.biorxiv.org/content/10.1101/2020.03.22.002386v1
In particular, since proteins encoded by ORF1ab, ORF3a, and ORF10 are postulated to target the hemoglobin B1 subunit, it would be interesting to see clinical investigations into drugs that target and interfere with these viral proteins, such as Pevonedistat, which is predicted to interact with the protein encoded by Orf10.
LikeLiked by 1 person
Interesting, it might leave a breakthrough to understand and treat COVID -19 mystery
LikeLike